2023 EULAR recommendationsー個々の推奨1-7ー
全般的指針から個々の推奨に移ります。ここでは1-7を説明します。意訳しているので、原文も張り付けておきます。Mycophenolateは日本で使えるMMFに置換しております。
ref) 2023 EULAR recommendations for SLE
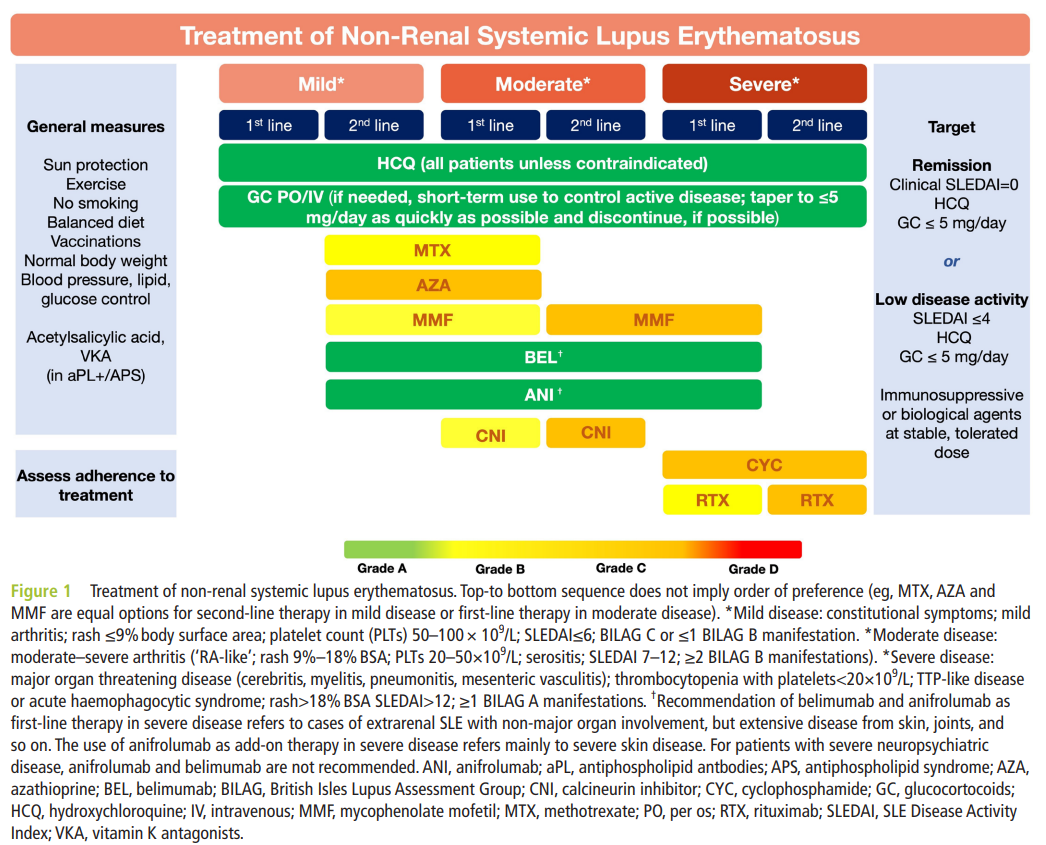
Individual recommendations
- Hydroxychloroquine is recommended for all patients (1b/A), unless contraindicated, at a target dose of 5 mg/kg real body weight/day (2b/B), but individualised based on risk for flare (2b/B) and retinal toxicity.HCQは禁忌でない限り全患者に推奨される(1b/A)。5mg/kg理想体重をターゲットとして(2b/B)。しかし個々の再燃リスク(2b/B)と網膜毒性に応じて。
HCQ is the mainstay of treatment for patients with SLE and the current SLR extended the existing body of evidence regarding the multiple beneficial effects of HCQ in various aspects of the disease. In the 2019 recommendations, emphasis was placed on the specification that HCQ dose ‘should not exceed 5 mg/kg real body weight/day’, in view of data which suggested a higher than previously thought risk for retinal toxicity by the use of more sensitive screening techniques.24 A recent observational study assessed the risk for flares in relation to HCQ dose during the previous 6-month period and found an almost twofold risk for any flare for doses ≤5 mg/kg/day (vs >5 mg/kg/day), increased to more than sixfold for moderate or severe flares, with a threshold dose calculated near 5 mg/kg/day.25 Until more data become available regarding benefit–risk relationship of different doses, it was decided that the HCQ target dose remains at 5 mg/kg/day; however, this should be individualised based on risk for flare and retinal toxicity, with patients at higher risk for retinal toxicity (kidney disease, preexisting macular or retinal disease, tamoxifen use) being candidates for closer ophthalmologic follow-up. In selected patients and circumstances (eg, moderate or severe disease), initial HCQ dose higher than 5 mg/kg/day (but not exceeding 400 mg/day) may be used, followed by lowering of the dose to within range once the patient has improved. In addition, Task Force members suggested the use of monitoring HCQ blood levels to guide the optimal dose for each patient and assess for possible non-adherence to therapy, based on studies suggesting that HCQ whole blood levels may reflect patient adherence to treatment.26 Although a universal recommendation for HCQ blood level monitoring would be impractical, it can nevertheless be used to guide dosage adaptations, in settings where it is available. Finally, the use of antimalarials other than HCQ was discussed mainly by non-European panellists, to address the issue of potential limited availability or higher cost of HCQ in some countries. In such settings, chloroquine may be used as an alternative, bearing in mind that it may be more toxic than HCQ (mainly for retinal toxicity).27 Finally, quinacrine can be considered in patients with cutaneous manifestations and HCQ-induced retinopathy. The statement on HCQ was agreed on by 77.8% of participants following one round of amendments (the only statement where this was needed) and mean (SD) LoA was 9.21 (3.35).
HCQはSLE患者の治療の基本である。現在のSLRはHCQのSLEの様々な局面における多くの利益に関するエビデンスの量を拡大した。2019の推奨ではHCQの量を5mg/kg理想田いう鵜を超えないようにとわざわざ強調した。より感度の高いスクリーニング検査を用いると当初考えられていたよりも高いリスクであったという点から。最近の観察研究はHCQの過去6か月の投与量と再燃のリスクとの関連を評価し、5mg以下は>5mgと比べ任意の再燃リスクが約2倍高いこと、中等度から重症の再燃は>6倍高いことが分かった。異なる量での利益とリスクとの関連に関するデータが入手できるようになるまで、HCQ投与量のターゲットは5mg/kgのままにすることが決定された;しかし、これは個々の再燃リスクと網膜毒性のリスクに基づいて決められるべきである。腎炎、過去の黄斑・網膜疾患、タモキシフェンの使用のような網膜毒性のハイリスクの患者はより短期間の眼窩フォローを受ける候補となるといった具合に。初期量HCQ>5mg/kg(400mg以下)も、患者が一旦改善した範囲に低下させれば使用できる。
加えて専門委員会のメンバーはHCQ血中濃度の測定を用いる事を提案した。個々の患者の最適な投与量を調整する事とアドヒアランスのチェックのために。HCQ総投与量が治療アドヒアランスを反映するかもしれない事を示唆する研究に基づいて。HCQの血中濃度のモニタリングの世界的な推奨は現実的ではないであろうが、測定できるのであれば投与量の調節をガイドするために用いる事は可能である。最後にHCQ以外の抗マラリア薬の使用、、、(略)
- Glucocorticoids, if needed, are dosed based on the type and severity of organ involvement (2b/C), and should be reduced to maintenance dose of ≤5 mg/day (prednisone equivalent) (2a/B) and, when possible, withdrawn; in patients with moderate-to-severe disease, pulses of intravenous methylprednisolone (125–1000 mg per day, for 1–3 days) (3b/C) can be considered.GCは必要であれば臓器障害のタイプと重症度(2b/C)に基づいて調整され投与されるべき。維持量としてはPSN換算量5mg以下に減量されるべき(2a/B)。可能であれば中止すべき;中等症~重症の患者では125-1000mg/dのパルス療法を1-3日を考慮できる(3b/C)。
Minimisation of GC use, in view of their detrimental effects, was a major theme of discussion during the Task Force meetings. Numerous studies in the current SLR confirmed associations of different cut-offs for daily prednisone dose with adverse outcomes, most of which pointed to the threshold of 5 mg/day. Although a controlled trial of different GC tapering regimens or maintenance doses is still lacking in SLE, the Task Force elected to lower the ‘acceptable’ threshold of daily prednisone dose for maintenance treatment to maximum 5 mg/day prednisone equivalent, as compared with 7.5 mg/day in the 2019 recommendations. Ideally, one could envision the use of GC only as ‘bridging therapy’ in SLE, similar to rheumatoid arthritis (lowest possible dose for the shortest possible period), and the complete withdrawal of GC is the optimal target.GCを最小限にすることはその良くない効果の面から委員会の期間中、主に議論されたテーマでった。現在のSLRにおける無数の研究が異なる1日あたりのカットオフ値と有害事象との関連を確認した。そのほとんどが5mgの閾値を示唆するものであった。SLEにおいて異なるGC減量レジメンを評価した比較試験はないが、委員会はacceptableな1日PSN量として最大5mgを選出した。2019年の推奨では7.5mg/dであったが。理想的にはGCを可能な限り少ない量を可能な限りの短期間で、というRAと同じようにbridging therapyだけのGC使用を思い描くことができるかもしれないし、完全な中止が理想的なターゲットである。
Intravenous pulses of methylprednisolone (MP) of various doses (depending on disease severity and patient weight) capitalise on the immediate non-genomic effects of GC,28 and may allow for a faster tapering of per os (PO) GC.29 Importantly, pulse IV MP has not been linked to certain established GC-related harms, like avascular necrosis.30 Initial PO dose also depends on disease severity; a retrospective study in 206 patients with LN using propensity score matching found higher rates of 1-year complete response in patients who started with ≥40 mg/day compared with those who started with≤30 mg/day, without increased risk for GC-related damage.31 A smaller study in non-renal lupus had found similar reduction in disease activity at 1 year and higher risk of damage with starting dose >30 mg/day versus ≤30 mg/day.32 Despite these discrepancies, most panellists agreed that it is the chronic exposure to GC that confers the major risk, and the statement received 96.3% agreement and mean (SD) LoA was 9.57 (0.77).MPの静脈パルスは重症度と体重に応じて様々な投与量でGCの急速なnon-genomic effectを利用したものでり、内服GCの急速な減量を可能にするかもしれない。大切なこととしてパルスIVMPはある特定のGC関連として確立した有害事象に関連していないことである(無血管性骨壊死のような)。初期の内服量は重症度にも依存する;PSMを用いたretrospective研究でLN 206例において1年後のCRは40m議場で開始した患者において30mg以下で開始した患者と比べ高く、GC関連ダメージのリスクは増えなかった。同様に小さな研究では非腎症において>30mgで開始した場合1年後の疾患活動性の低下は30mg以下と比べ同等であった。これらの乖離があるものの、ほとんどのパネリストは慢性のGC暴露こそがメジャーなリスクに相当する事に同意し、生命は96.3%の賛成とLoA=9.57(SD 0.77)に至った。
- In patients not responding to hydroxychloroquine (alone or in combination with glucocorticoids) or patients unable to reduce glucocorticoids below doses acceptable for chronic use, addition of immunomodulating/immunosuppressive agents (eg, methotrexate (1b/B), azathioprine (2b/C) or mycophenolate (2a/B)) and/or biological agents (eg, belimumab (1a/A) or anifrolumab (1a/A)) should be considered.HCQに反応しない患者(単独orGCと併用して)やGCを慢性使用に容認できる量以下まで減量できない患者では、免疫調整・抑制剤and/or生物製剤を考慮すべき。MTX, 1b・B;AZ 2b・C;MMF, 2a/B;Belimumab 1a/A, Anifrolumab 1a/A
This statement emphasises the value of conventional and biological immunomodulatory/immunosuppressive drugs for the control of the disease and facilitation of GC tapering and withdrawal. Since no new, high-quality data emerged in the past 4 years regarding conventional immunosuppressive drugs, deliberations regarding this statement focused on two main issues: (1) inclusion of anifrolumab, following its approval in 2021,33 34 as well as belimumab,35 as biological agents with proven efficacy in controlling disease activity, reducing flares, and allowing for GC dose reduction. In the recommendation, there is no hierarchy in the choice between anifrolumab and belimumab, as the two drugs have not been compared in a head-to-head trial and their approval was the result of RCTs in similar extrarenal SLE populations. The panel noted that there are more than 10 years of real-life clinical experience with belimumab, while no real-life data for anifrolumab had been published by the time of the SLR completion. (2) The positioning of biological agents in relation to conventional immunosuppressive drugs for the treatment of SLE. For the latter point, while considerations from specific countries, healthcare settings and biological reimbursement policies have to be taken into account, most panellists agreed that prior use of a conventional immunosuppressive drug (MTX, AZA, mycophenolate mofetil or mycophenolic acid (henceforth combined referred to as ‘mycophenolate’, see online supplemental table 1 for details), leflunomide36 or others) should not be mandatory for initiating anifrolumab or belimumab. この声明は疾患活動性のコントロールとGC減量・中止の促進の点におけるconventionalとbiologicalな免疫調整・抑制剤の価値を強調したものである。Conventionalな免疫抑制剤については新しい質の高いデータが過去4年出ていないため、この声明に関する審議は以下の2点にフォーカスした:①疾患活動性のコントロールと再燃抑制、GC減量を可能にする事に関する効果が示された生物製剤として、belimumabと同様に2021年に承認されたanifrolumabを含む事。推奨ではanifrolumabとbelimumabの優劣はつけてない。いずれもhead-tohead試験は行われておらず、それらの認可は腎炎以外の同様のpopulationにおけるRCTの結果に基づくため。委員会はBelimumabについては>10年のreal-lifeの臨床経験があるが、anifrolumabに関してはreal-life dataがない事がSLR完了の時点えなかったことを注釈した。➁SLE治療における生物製剤のconventionalな免疫抑制剤に関連した位置づけ。biologicsについて、特定の国々、ヘルスケアの状況と生物製剤の返済ポリシーを考慮に入れなければならない、ほとんどのパネリストはconventional IS (MTX, AZ, MMF, MPA, LEF, or other)の先行使用をanifrolumab or belimumabよりも前に強制すべきではない事に同意した。Of note, this is unchanged from the 2019 recommendations. The rationale driving this statement was that, despite their substantially higher cost, approved biological drugs have proven their efficacy in high-quality RCTs, while such data are lacking for conventional immunosuppressive drugs, which continue to be used based on rheumatologists’ long-term real-life experience. This recommendation received 84.6% agreement and mean (SD) LoA was 9.32 (0.91).
特にこれは2019年から変わっていない。この声明をするに至った合理性はそのコストにも関わらず、質の他愛RCTによってそれらの効果が示されているからだ。一方、長期のreal-lifeの経験に基づいて使用され続けられているconventionalなISではそのようなデータはない。
- In patients with organ-threatening or life-threatening disease, intravenous cyclophosphamide (2b/C) should be considered; in refractory cases, rituximab (2b/C) may be considered.
Due to its gonadal toxicity, high-dose IV CYC is reserved for severe cases of lupus, in which it may be considered owing to its high efficacy. Rituximab (RTX) is used off-label in SLE and is recommended in circumstances37 where other drugs have failed, with notable exceptions (eg, immune cytopenias, see below) where it can be used earlier. Although a universal definition of refractory disease is lacking in SLE, it is conceived as disease not responding to different classes of immunosuppressive medications. The combination of RTX with CYC had been used in early studies of RTX,38 but additional benefit has not been confirmed39 and this combination comes at the expense of an increased risk for infections. Sequential strategies of RTX followed by belimumab are being explored, but more data are needed.40 41
Patients not responding to any of the aforementioned therapies might be offered other options, such as plasma exchange,42 haematopoietic stem cell transplantation43 or experimental therapies. In 2022, the use of CAR T-cells in five patients with severe, refractory SLE was published with encouraging results, yet RCTs and more long-term data are needed.44 Agreement for this recommendation was 100%, with mean (SD) LoA 9.38 (0.99). 生殖器毒性のため高用量IV CYCは重症例に温存される。重症例における高い有効性のため。RTXはSLEではoff-labelの使用となり、その他の薬剤が失敗に終わった場合に推奨される。ただし早期に使用できる顕著な例外はあるが(以下に示す血球減少など)。難治例の普遍的な定義がないため、異なる免疫抑制剤に反応しない患者がこれに相当する。RTXとCYCの兵四湯はRTXの早期の試験で使用されたが、追加的な利益は確認されなかった。この併用あ感染症リスクの上昇という代償をもたらす。続くbelimumabを後療法としたRTXの戦略が探求されているが、データがまだ少ない。上述の治療に反応しない患者は血漿交換療法や骨髄移植のようなその他の治療を提供されてもよいかもしれない。2022年、CAR-T cellsが重症難治のSLE患者5例で勇気づけられる結果をもって出版された。RCTやさらなる長期データが必要である。同意度は100%で、LoA 9.38.
- Treatment of active skin disease should include topical agents (glucocorticoids, calcineurin inhibitors) (2b/B), antimalarials (hydroxychloroquine, chloroquine) (1a/A), and/or systemic glucocorticoids (4/C) as needed, with anifrolumab (1a/A), belimumab (1a/B), methotrexate (1b/B), or mycophenolate (4/C), considered as second-line therapy.皮膚疾患の治療には局所治療(GC、CNI)(2b/B)、抗マラリア薬(HCQ, QC)1a/A、必要に応じて全身性GC(4/C)を含むべき。anifrolumab1a/A、belimumab1a/B、MTX1b/B、MMF4/Cを第二選択として。
For the treatment of active skin disease in SLE, few new data have emerged since the 2019 recommendations, and a significant body of evidence continues to originate from studies in patients with cutaneous lupus erythematosus. Recommended first-line treatment (topical agents, antimalarials and/or systemic GC) has not changed in the statement. HCQ is the antimalarial of choice, but chloroquine may be used in the settings discussed earlier.45 Quinacrine (mepacrine) may also be used in cases of inadequate response or toxic retinopathy, as add-on to HCQ or alternative therapy, respectively,46 but its use is limited by frequent intolerance and unavailability in many countries.SLEの活動性皮膚疾患の治療については新しいデータは2019推奨からほとんど出ていない。CLEの患者から大量のエビデンスが出続けている。推奨される第一選択(局所治療、抗マラリア薬、全身性GC)は生命において変わっていない。HCQは抗マラリア薬の選択肢となるが、CQは早期に議論された状況では使用しうるかもしれない。Quinacrine略
For the ~40% of patients not responding to first-line therapy,47 comparative studies among existing immunosuppressive drugs are lacking. Despite this paucity, recommended second-line drugs have partly changed from 2019, because the Task Force decided to recommend drugs more familiar to rheumatologists (such as MTX or mycophenolate, instead of dapsone or retinoids). A small retrospective study in 73 patients with refractory CLE to first-line therapy found similar response rates (~65%) between MTX and mycophenolate.48 Anifrolumab and belimumab have both shown efficacy in mucocutaneous manifestations of SLE,49 50 although only anifrolumab has used the Cutaneous Lupus Area and Severity Index in its clinical programme, whereas belimumab has reported responses according to the general instruments SLEDAI and BILAG (hence, the designation B in the Grading of Recommendation, despite positive RCT data). Importantly, the list of recommended drugs is indicative and other treatments may be considered as second-line or third-line options, including dapsone, retinoids, CNI, AZA, CYC and RTX, ideally in collaboration with dermatologists experienced in the treatment of CLE. Finally, thalidomide and, more recently, lenalidomide, are effective in various subtypes of cutaneous lupus,51 52 and lenalidomide has a lower risk for polyneuropathy than thalidomide; however, they should both be reserved for patients that have failed multiple previous agents, and with the utmost caution in women of reproductive age. 40%までの患者が第一選択に反応しないが、このような患者において現存するISの比較研究はない。これにもかかわらず推奨される第二選択は2019年から一部変わった。委員会はリウマトロジストが慣れている薬剤(dapsoneやretinoidsよりもMTXやMMF)を推奨することに決めた。初期治療に抵抗性であった難治性CLE73例の小さな研究はMTXとMMFで同等の反応を示した(~65%)。AnifrolumabとbelimumabはともにSLEの皮膚粘膜症状に有効性を示したが、CLASIをアウトカムとして評価したのはanifrolumabだけであるが、belimumabはSLEDAI、BILAGに基づいた反応を報告している(したがってRCTのpositive dataにもかかわらず推奨のグレードはBとした)。重要なことに推奨される薬剤のリストは指標であり、その他の治療も第二、第三選択で考慮されてもよい;これにはdapsone、retinoids、CNI、AZ、CYC、RTXが夫君れ、理想的にはCLE治療の経験が豊富な皮膚科医と併診の上。最後にサリドマイド、最近ではLEFも様々なCLEのサブタイプに有効であるが、LEFはサリドマイドに比べポリニューロパチーのリスクが低いが、生殖可能年齢の女性では最大の注意が必要。This recommendation was agreed on by 96.3%, mean LoA (SD) was 9.35 (1.06).
- In active neuropsychiatric disease attributed to SLE, glucocorticoids and immunosuppressive agents for inflammatory manifestations (1b/A) and antiplatelet agents/anticoagulants for atherothrombotic/antiphospholipid antibodies (aPL)-related manifestations (2b/C) should be considered.SLEに起因する活動性NPでは炎症病態にはGCと免疫抑制剤を(1b/A)、動脈硬化性・抗リン脂質抗体関連症状には抗血小板薬・抗凝固薬を考慮すべき(2b/C)
This recommendation has remained unchanged, since no new data have emerged during the last 5 years that would deem a modification of the recommendation appropriate.53 Approach to a patient with possible neuropsychiatric SLE should follow the general principles as in the general population and symptomatic treatment as per individual manifestation should be considered. Attribution of a neuropsychiatric manifestation to SLE is challenginf and published attribution models may be used in doubtful cases.54 55 For severe inflammatory manifestations (eg, myelopathy, acute confusional state), potent immunosuppressive agents, like CYC or RTX,56 should be preferred. For the approved biological drugs anifrolumab and belimumab, there is a paucity of evidence regarding their efficacy in neuropsychiatric manifestations, because patients with active, severe forms of such manifestations were excluded from the RCTs of both drugs, and under-represented in real-life use of belimumab. Anticoagulant treatment is mainly indicated in cases of cerebrovascular disease, such as ischaemic stroke associated with aPL, because its value in other manifestations is not clear. この推奨は変わっていない。過去5年、推奨の変更が適切と思われるような新しいデータは出ていないので。NPSLEの患者へのアプローチは一般人口における全般的な考え方に従うべきであり、個々の症状に応じた対症療法を考慮すべき。SLEによるNPへの帰属はchallengingであり、出版された帰属モデルを疑わしいケースには用いてもよい。炎症病態の重症例(脊髄炎、ACS)ではCYCやRTXのような強力な免疫抑制剤が好まれるべき。承認されたAnifrolumabとbelimumabにおいてはそれらのNPに対する効果に関するエビデンスがほとんどない。活動性で重症のそのような症状の患者はいずれの薬剤のRCTにおいても除外されていたためだ。 Belimumabでも実臨床における使用はまだ少ない。工業子役は主にaPLに関連した虚血性strokeのような脳血管疾患に指示される。その他の症状に対する価値はわかっていないため。Agreement on this recommendation was 96.3% and mean (SD) LoA was 9.68 (0.81).
- For acute treatment of severe autoimmune thrombocytopenia, high-dose glucocorticoids (including pulses of intravenous methylprednisolone) (4/C), with or without intravenous immunoglobulin G (4/C), and/or rituximab (2b/B), and/or high-dose intravenous cyclophosphamide (4/C), followed by maintenance therapy with rituximab (2b/B), azathioprine (2b/C), mycophenolate (2b/C), or cyclosporine (4/C), should be considered.重症の自己免疫性血小板減少症に対して高用量GC(mPSL静注パルスを含め)(4/C)を考慮すべき。IVIG (4/C), RTX (2b/B), high-dose iv CYC (4/C)を併用してもよい。 その後の維持療法としてはRTX (2b/B), AZ (2b/C), MMF (2b/C), or cyclosporine (4/C)を考慮すべき。
Similar to neuropsychiatric SLE, treatment of autoimmune thrombocytopenia in SLE also did not witness major developments since 2019; thus, the current recommendation reflects the principles outlined in the previous update. A platelet number of 20–30 000/mm3 is typically used as the cut-off, below which therapy is indicated. Therapy includes: (1) acute phase treatment with GC (including pulses of intravenous MP, followed by 0.5–0.7 mg/kg/day prednisone equivalent with tapering) with or without IVIG; RTX may also be used early in this setting,57 based also on the drug’s documented efficacy in idiopathic immune thrombocytopenia, (2) early use of immunosuppressive medications as GC-sparing agents; a small retrospective study showed that patients with SLE with immune cytopenias who relapsed had less often received concomitant immunosuppressive agents following treatment of the initial episode.58 More importantly, mycophenolate was shown to reduce relapse when used as first line in a RCT in patients with immune thrombocytopenia.59 While a similar RCT has not been performed in SLE, this study provided proof-of-concept for the first-line use of immunosuppressive medications in immune thrombocytopenia, a practice commonly followed by most rheumatologists . NPと同様、自己免疫性血小板減少症は2019年から大きな進歩がない;したがって現在の推奨は2019年版で要約された方針となっている。2-3万が典型的にはカットオフ値として使用され、それ以下が適応となる。治療には①急性期のGC治療(MPパルス後のPSN相当量0.5-0.7mg/kgを含め)±IVIG。RTXを早期に用いてもよい。過去の特発性免疫性血小板減少症IITにおける記述された有効性に基づいて。➁GC-sparingとして用いられる免疫抑制剤の早期の使用;小さな観察研究において、再発性の免疫性血球減少症のSLE患者は初期エピソードにおいて免疫抑制剤の使用がより少なかった。より重要な事はMMFは免疫性血小板減少症の患者におけるRCTにおいて第一選択で用いられたとき再発が少なかった事が示されている同様のRCTはSLEでhなされていないが、この研究は免疫性血小板減少症における免疫抑制剤の第一選択薬としての概念の証明を提供しており、多くのリウマチ専門医が一般的に従っているプラクティスである。Regarding choice of drug for maintenance therapy, there is no hierarchy between the recommended drugs, and this is left to the treating physician’s discretion. In cases refractory to these drugs, thrombopoietin receptor (TPO) agonists and splenectomy are options; although the two modalities have not been formally compared in SLE and data mainly come from observational studies,60 it seems reasonable to use TPO agonists prior to splenectomy, given the possible complications and long-term sequelae of the latter. However, it should be considered that TPO agonists have been associated with a higher risk of thromboembolic events, therefore their use should be avoided in aPL-positive patients.61 Fostamatinib, a spleen tyrosine kinase inhibitor, is approved for the treatment of chronic immune thrombocytopenia, but has not been tested in SLE. Similar treatment options (excluding TPO agonists) pertain to SLE autoimmune haemolytic anaemia. 維持療法の治療薬の選択については推奨された薬剤の間でヒエラルキーはない。これは治療する意思の裁量に委ねられている。これらの薬剤に抵抗性のケースではthrombopoietin receptor (TPO) agonists と脾摘が選択しとなる;この2つの方法はSLEで正式に比較されたわけではなく、SLEでは主に観察研究から来ているにすぎないが、TPO刺激薬を脾摘よりも先に使用する事は脾摘の合併症と長期的な後遺症を考慮するとreasonableであろう。しかしTPO刺激薬は血栓塞栓症のリスク上昇と関連していることも考慮されるべき。従ってその使用はaPL要請患者では避けられるべき。Fostamatinib、脾臓チロシンキナーゼ阻害薬が慢性免疫性血小板減少症の治療に承認されたが、SLEではテストされていない。同様の治療選択(TPO刺激薬を除く)がSLEの自己免疫性溶血性貧血にも当てはめることができる。This recommendation received 92.6% agreement and mean (SD) LoA was 9.48 (0.86).
ps;
2023 EULAR recommendations for SLEー全般的指針ー - リウマチ膠原病のQ&A (hatenablog.com)
2023 EULAR recommendationsー個々の推奨8-12ー - リウマチ膠原病のQ&A (hatenablog.com)